
Find out more about Bioperfectus COVID-19 Ag Rapid Test on Africa Medical Supplies Platform (launched by African Union) via searching “bioperfectus” at
https://amsp.africa/
As the COVID-19 pandemic is widely spreading in Africa, the Africa CDC had issued the Interim Guidance on the Use of Rapid Antigen tests for COVID-19 Response on 16 December as reference for clinicians, laboratory professionals, policymakers and other stakeholders. It suggests that the Member States of Africa Union including Central Africa, Eastern Africa, Northern Africa, Southern Africa, Western Africa should aim to test all individuals with symptoms consistent with COVID-19 as quickly as possible, and it states that antigen tests are access to enlarge testing scale and enable timely reports for persons in specific settings despite whether they have symptoms.
According to the guidance, COVID-19 rapid antigen tests (COVID-19 Ag-RDTs) is recommended for individuals with symptoms, high-risk populations including healthcare workers and contacts irrespective of symptoms in settings where nucleic acid amplification testing (NAAT) is not available or the turnaround time for result delivery is prolonged due to overloads or other reasons.
Target populations of COVID-19 Ag-RDTs according to the guidance:
- Individuals with symptoms
- Frontline healthcare workers and essential workers (symptomatic and asymptomatic)
- High-risk populations in areas with confirmed/suspected outbreak (includes the elderly, people with comorbidities, and populations in closed settings such as prisons, care homes, etc)
- Contacts of confirmed cases (symptomatic and asymptomatic)
Source: Interim Guidance on the Use of Rapid Antigen tests for COVID-19 Response
COVID-19 Ag-RDTs is also recommended in the guidance for providing rapid detection and epidemic management in clusters or settings (e.g., workplaces, educational institutions, correctional facilities) with suspected or confirmed outbreaks.
The guidance has also suggested clear routes in the regard of follow-up strategy of positive and negative results indicated by COVID-19 Ag-RDTs.
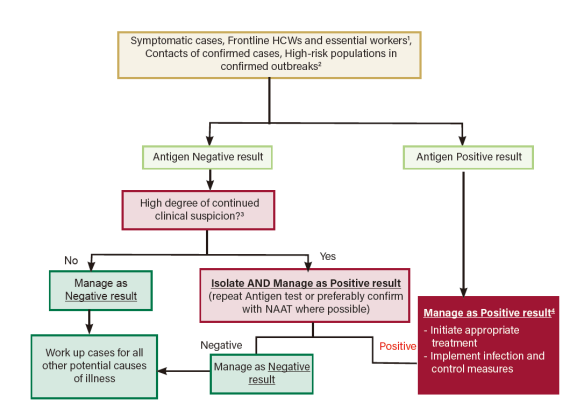
Figure 1: Algorithm when testing populations with higher suspicion of positivity.
Source:Interim Guidance on the Use of Rapid Antigen tests for COVID-19 Response

Figure 2: Algorithm for general screening of persons (irrespective of symptoms) in settings with unknown or low community transmission, including in schools, workplaces, ports of entry or houses of worship, etc.
Source: Interim Guidance on the Use of Rapid Antigen tests for COVID-19 Response
Last but not least, the guidance emphasizes that reliable test results are essential to implement COVID-19 Ag-RDTs in population screening. In addition, all results should be included in the national surveillance data, and RT-PCR and COVID-19 Ag-RDT test results should be disaggregated for better oversight of the testing programs in where it’s possible.
References
- Interim Guidance on the Use of Rapid Antigen tests for COVID-19 Response
https://africacdc.org/download/interim-guidance-on-the-use-of-rapid-antigen-tests-for-covid-19-response/#
- World Health Organization. COVID-19 Strategy Update. 14 April 2020 (https://www.who.int/publications/i/item/covid-19-strategy-update---14-april-2020, accessed 24 November 2020).
- FIND. Rapid diagnostic tests for COVID-19 (https://www.finddx.org/wp-contenthttp://shuo-shi.oss-cn-beijing.aliyuncs.com/uploads/2020/05/FIND_COVID-19_RDTs_18.05.2020.pdf, accessed 24 November 2020).
- World Health Organization. COVID-19 Target product profiles for priority diagnostics to support response to the COVID-19 pandemic v.1.0. 28 September 2020 (https://www.who.int/publications/m/item/covid-19-target-product-profiles-for-priority-diagnostics-to-support-response-to-the-covid-19-pandemic-v.0.1, accessed 24 November 2020)
- Chin ET, Lo NC, Huynh BQ, Murrill M, Basu S. Frequency of routine testing for SARS-CoV-2 to reduce transmission among workers. medRxiv [Preprint]. 2020 May 6:2020.04.30.20087015. doi: 10.1101/2020.04.30.20087015. PMID: 32511523; PMCID: PMC7273291.
- World Health Organization. Public health criteria to adjust public health and social measures in the context of COVID-19: annex to considerations in adjusting public health and social measures in the context of COVID-19, 12 May 2020 (https://apps.who.int/iris/handle/10665/332073, accessed 24 November 2020)

